Found 18 Results

Altman Sidney
Sidney Altman was born in Montreal, Canada, in 1939.
In 1989, he was awarded the Nobel prize in chemistry “for discovering the catalytic nature of RNA.
Altman discovered that RNA and DNA do not only carry the genetic code, but have also the ability to start and control chemical reactions.

Baeyer Adolph Von
Adolph Von Baeyer was born in 1835 in Berlin.
He received the 1905 Nobel prize in chemistry for advancing “organic chemistry and the chemical industry, through his work on organic dyes and hydro-aromatic compound.”
Adolph Von Baeyer passed away in 1917.

Berg Paul
Paul Berg was born in 1926 in New-York.
He received the 1980 Nobel prize in chemistry “for his fundamental studies of the biochemistry of nucleic acids”, including the first recombination of genes.

Brown Herbert
Herbert Brown was born in London, in 1912 and since early childhood had to provide for his poor family. That did not stop him from succesfully completing his education.
In 1979 Herbert Brown received the Nobel prize in chemistry “for developing the use of boron in organic synthesis.”
Brown’s methods are used worldwide as basic procedures in laboratory work.

Calvin Melvin
Melvin Calvin was born in the United States of America in 1911.
Melvin Calvin received the 1961 Nobel prize in chemistry, “for his research on the carbon dioxide assimilation in plants,” in which he detailed the chemical reactions participating in the process of photosynthesis.

George Olah
Prof. George Olah’s research terminology includes “carbocations” and “superacids”, terms that in order to fully understand you literally need to spend a life time in a lab.
Let us, then, convert these terms to easy words any layman can relate to: fuel substitute. In other words – “Methanol Economy”.
Methanol is the simplest alcohol, that can be produced from sugar canes, corn or wood. It’s main advantage is being an alternative for oil as an energy source for transportation. It is also environment-friendly, unlike gasoline and diesel that emit carbon dioxide into the atmosphere, thus increase the greenhouse effect and the danger of turning the entire planet into a gigantic hot tanning booth.
George Olah, a Hungarian Jew who was born in 1927 in Budapest, managed to cross the iron curtain in 1956 and came to the United States. Moving from cold communist Hungary to warm liberal California must have agreed with him, for he became one of the most prolific scientists of our time. He registered 120 patents, published 1,400 academic articles, over 20 books, including one on the “Methanol Economy”, won dozens of prestigious awards, 15 honoris causa degrees, and in 1994 – the Nobel Prize in economics.

Gilbert Walter
Walter Gilbert was born in 1932 in Boston.
He received, together with Frederic Sanger, the 1980 Nobel prize in chemistry “for their contributions concerning the determination of base sequences in nucleic acids”.
Gilbert’s achievements made possible the diagnosis and prevention of hereditary diseases.

Haber Fritz
Fritz Haber was born in Breslau, Poland in 1868. At the age of 25, he began studying organic chemistry, but he was drawn to applied physical chemistry. In 1896, he was named a professor at Karlsruhe and from the year 1911, headed the Kaiser Wilhelm Institute of physical chemistry.
During World War I Haber, helped develop the German chemical industry for war purposes and also played a role in developing gases for chemical warfare. Haber left the Jewish faith and was not harassed by the Nazis when they rose to power. However, in 1933, he was forced to flee to England after refusing to fire his Jewish assistants. He then went to Switzerland where he continued his research.
In 1918, Fritz Haber was awarded the Nobel Prize in Chemistry “for the synthesis of ammonia from its elements”. Ammonia is an extremely important raw chemical material. Today, cooling and air-conditioning systems are among its many uses.
Haber developed a system for producing ammonia, and with the help of Carl Bosch, also found new avenues for its industrial use. Haber’s developments continue to serve industry until this very day.
Fritz Haber died in 1934.

Harry Kroto
25 years ago, Harry Kroto discovered a carbon structure he chose to name buckyball, because of its soccer ball shape. However the official name was Fullerene, in honor of the gigantic domes planned by Buckminster Fuller.
Buckminsterfullerene (or buckyball) is a spherical Fullerene molecule with the formula C60. It has a cage-like fused-ring structure which resembles a soccer ball, made of twenty hexagons and twelve pentagons, with a carbon atom at each vertex of each polygon and a bond along each polygon edge.
Buckyball has become a star overnight – it is the logo of modern chemistry, and was even picked as the national molecule of the state of Texas.
Due to its unique features, its strong and stable structure, and the high conductivity, it has become the star of nanotechnology.
In the near future we may enjoy extremely light computers made of fullerene, extra strong batteries, nanorobots to be inserted into the body and fight diseased and maybe even an elevator from earth to space bases using fullerene cables.

Hevesy George Von
George Von Hevesy was born in 1885 in Hungary. He studied at the universities of Budapest and Freiburg and earned a doctorate at the age of 23. In 1911, he moved to Manchester University, where he conducted research on isotopes. After returning to Budapest University, he was appointed professor, but was dismissed shortly thereafter because he was a Jew. He then moved to Denmark and worked in the Niels Bohr Institute and eventually settled in Sweden.
George Von Hevesy was awarded the 1943 Nobel Prize in Chemistry “for his work on the use of isotopes as tracers in the study of chemical processes”.
His paper on radioactive labeling and tracing forms the basis of all radioactive isotopic tracer techniques used in biology, metallurgy, medicine and analytical chemistry. In 1922, he discovered the element Hafnium, which provided one of the first pieces of supporting evidence for the modern theory of atomic structure.
Von Hevesy also developed an analytical technique using accelerated neutrons, which became the most important and precise method available for determining the purity of substances.
George Von Hevesy passed away in 1966.

Hoffmann Roald
Roald Hoffmann was born in Zloczow, Poland, in 1937. His father was killed during the Second World War, and he and his family became refugees, moving from one displaced person camp to another until 1949, when they eventually settled in the United States.
These are the words of Hoffmann, the Holocaust survivor, the scientist and the poet describing his youth: “The man for whom everything came easy came from an immigrant family and didn’t own a book until he was 16.”
Hoffmann studied chemistry at Columbia University, then at Harvard where he received his doctorate in 1962. He moved to Cornell University in 1965. His field of research dealt with the reactions between atoms and molecules, but he also became interested in philosophy, art and symbolism.
Roald Hoffmann was awarded the Nobel Prize in Chemistry in 1981 for his part in what became known as the Woodward-Hoffmann Rules. These rules provide a rational explanation for much of what occurs in organic chemistry. They make it possible to predict which chemical reactions can take place easily and which are impossible to achieve.
Hoffmann wrote in one of his books: “In the Jewish tradition from which I stem, there is a concept called ‘TIKUN OLAM’, which means that whatever is done by man can be changed. We are compelled by our nature to create, but we can choose to fashion the world according to the good or according to the evil that is within us.”

Klug Aaron
Aaron Klug was born in 1926 in Lithuania; he studied in South Africa for his B.Sc and M.Sc. degrees. He received his Ph.D. in chemistry from Cambridge University in England, and in 1954 began working at Birckback College in London. From 1962, he began directing the MRC Laboratory of Molecular Biology.
In 1982, Aaron Klug was awarded the Nobel Prize in Chemistry “for his development of crystallographic electron microscopy and his structural elucidation of biologically important nuclei acid-protein complexes”.
Klug developed a method for deciphering the spacial structure of macromolecules by means of photographs taken with an electronic microscope. He also provided a theoretical basis for the reliability of his method.
In his research, Klug attempted to understand the connection between the organizing rules of macromolecules and their capability to perform various biological functions. In that, he laid the foundation for a fruitful research assisting in the comprehension of the macromolecule in fields such as viruses, the deciphering of the spacial structure of RNA-transfer and the structure of chromosomes bearing genetic information.

Marcus Rudolph
Rudolph Marcus was born in 1923 in Montreal, Canada. He received his doctorate from McGill University in 1946, and later moved to the United States. He also worked in England and China.
In 1992, Rudolph Marcus was awarded the Nobel prize for Chemistry “for his contributions to the theory of electron transfer reactions in chemical systems”.
Marcus’s work is related to the reduction of oxidation and it enables a deep understanding of many chemical processes such as photosynthesis, the electrical conductivity of polymers and corrosion. Itis conceivable that, in the futurem Marcus’s theory will support the discovery of a method of slowing down chemical reactions, which is impossible at present.
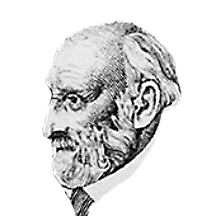
Moissan Henri
Henri Moissan was born in Paris in 1852.
He received the 1906 Nobel prize in chemistry “For the investigation and isolation of the element Fluorine, and for the invention of the electric furnace called after him.”
Henri Moissan passed away in 1907.

Perutz Max
Max Perutz was born in Vienna, Austria, in 1914.
He received the 1962 Nobel prize in chemistry together with John Kendrew, “For their studies of the structures of globular proteins”, Hemoglobin and Myoglobin.

Stein William
William Stein was born in New York in 1911. He studied chemistry and his research led him to develop ways of identifying amino acids and of localizing structures.
In 1972, he was awarded the Nobel prize in chemistry, together with Stanford Moore, “for their contribution to the understanding of the connection between the chemical structure and catalytic activity of the active center of the ribonuclease molecule.”
William Stein died in 1980.
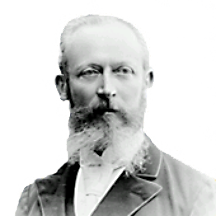
Wallach Otto
Otto Wallach was born in 1847 in Koenigsberg, Germany. He studied chemistry at the University of Goettingen. In 1870 he moved to Bonn, where he was appointed Professor of Chemistry in 1876. In 1889 he returned to the University of Goettingen and served as professor and director of the Chemistry Institute until he retired in 1915. Wallach continued his research until the age of 80.
In 1910 Otto Wallach won the Nobel prize in Chemistry “In recognition of his services to organic chemistry and the chemical industry by his pioneer work in the field of alicyclic compounds.”
Wallach’s main contribution was in laying the groundwork for the identification of the terpenes – a group of natural and synthetic hydrocarbons – and in determining the characteristics of camphor. camphor is a natural substance with a sharp aromatic smell, used in medicine for its sterilizing and anesthetic capabilities, and in the cosmetics industry for its pleasant aroma.
Wallach’s main contribution was in laying the groundwork for the identification of the terpenes – a group of natural and synthetic hydrocarbons – and in determining the characteristics of camphor. camphor is a natural substance with a sharp aromatic smell, used in medicine for its sterilizing and anesthetic capabilities, and in the cosmetics industry for its pleasant aroma.
Otto Wallach passed away in 1931.

Willstaetter Richard
Richard Willstaetter was born in Karlsruhe, Germany, in 1872. He studied chemistry and in 1902 became a professor at the University of Munich. In 1905 he moved to the Zurich Technicum and in 1915 he was appointed director of the Imperial Institute of Chemistry in Berlin. He returned to the University of Munich in 1916, replacing his late teacher, Adolf von Baeyer.
In 1925 Willstaetter resigned his teaching position in protest over anti-Semitism in the teaching faculty. With the rise of Nazism his property was confiscated, but he was allowed to move in 1939 to Switzerland, where he lived until his death in 1942.
Richard Willstaetter was awarded the Nobel prize for chemistry in 1915, “for his researches on plant pigments, especially chlorophyll.”
His chief contribution was in the acknowledgment of the biological significance of natural substances. He decoded the structure of various alkaloids, particularly those of the tropine group. His greatest achievement was the decoding of the complex structure of chlorophyll and the process of photosynthesis. Willstaetter was a pioneer in enzyme research, which he viewed as the key to understanding the complex processes of life.
Richard Willstaetter advocated liberalism and humanism and he saw in their realization the moral value of science, which, in his view, was designed to right the wrongs of human society.
free text
Select a Nobel category:
- Chemistry
- Economics
- Literature
- Medicine
- Peace
- Physics
Select first letter of winner's name:
- A
- B
- C
- D
- E
- F
- G
- H
- I
- J
- K
- L
- M
- N
- O
- P
- Q
- R
- S
- T
- U
- V
- W
- X
- Y
- Z
Search Results
Found 0 Results
No search results

region on map
